The Cost Burden of Multiple Sclerosis in the United States a Systematic Review of the Literature
Abstract
Background
Although the economic burden of multiple sclerosis (MS) in high-income countries (HICs) has been extensively studied, information on the costs of MS in low- and middle‐income countries (LMICs) remains scarce. Moreover, no review synthesizing and assessing the costs of MS in LMICs has notwithstanding been undertaken.
Objective
Our objective was to systematically identify and review the price of illness (COI) of MS in LMICs to critically assess the methodologies used, compare cost estimates across countries and by level of illness severity, and examine cost drivers.
Methods
We conducted a systematic literature search for original studies in English, French, and Dutch containing prevalence or incidence-based price information of MS in LMICs. The search was conducted in MEDLINE (Ovid), PubMed, Embase (Ovid), Cochrane Library, National Health Service Economic Evaluation Database (NHS EED), Econlit, and CINAHL (EBSCO) on July 2020 without restrictions on publication date. Recommended and validated methods were used for information extraction and analysis to brand the results of the COI studies comparable. Costs were adjusted to $US, twelvemonth 2019 values, using the Globe Bank purchasing power parity and inflated using the consumer price index.
Results
A full of 14 studies were identified, all of which were conducted in upper-middle-income economies. Eight studies used a bottom-up approach for costing, and six used a top-down approach. Four studies used a societal perspective. The total annual cost per patient ranged betwixt $US463 and 58,616. Costs varied across studies and countries, mainly because of differences regarding the inclusion of costs of disease-modifying therapies (DMTs), the range of cost items included, the methodological choices such as approaches used to approximate healthcare resource consumption, and the inclusion of informal care and productivity losses. Characteristics and methodologies of the included studies varied considerably, especially regarding the perspective adopted, cost data specification, and reporting of costs per severity levels. The total costs increased with greater disease severity. The price ratios between dissimilar levels of MS severity within studies were relatively stable; costs were around ane–one.v times higher for moderate versus mild MS and about 2 times college for severe versus mild MS. MS drug costs were the main toll driver for less astringent MS, whereas the proportion of direct non-medical costs and indirect costs increased with greater disease severity.
Conclusion
MS places a huge economic burden on healthcare systems and societies in LMICs. Methodological differences and substantial variations in terms of absolute costs were found between studies, which made comparison of studies challenging. However, the price ratios beyond different levels of MS severity were similar, making comparisons betwixt studies past disease severity feasible. Cost drivers were mainly DMTs and relapse treatments, and this was consistent beyond studies. Yet, the distribution of cost components varied with disease severity.
FormalPara Central Points for Decision Makers
| Multiple sclerosis (MS) imposes a significant economic burden in depression- and center‐income countries (LMICs). The full costs of the disease increase with disease severity. Costs of MS drugs dominate in less severe disease, whereas the proportion of direct not-medical costs and indirect costs increases with illness severity. |
| Substantial variations in MS costs were found between studies in LMICs, which made comparison of studies challenging. Yet, the cost ratios across dissimilar levels of MS severity were similar. Therefore, future cost-of-affliction (COI) studies of MS in LMICs should include all MS-related toll categories and report on cost per affliction severity level as MS costs significantly depend on Expanded Disability Status Calibration categories. |
| COI studies should clearly define the perspective and data sources used. Methodologies adopted to judge healthcare resource consumption, informal care and productivity losses should exist well-divers and in alignment with the country's own healthcare system and specifications as a marking of the reliability of the COI estimate. |
Introduction
Multiple sclerosis (MS) is an inflammatory and demyelinating disease of the central nervous system that affects 2.8 million people worldwide and has a prevalence of 36 per 100,000 people [1]. It is the leading cause of non-traumatic disability in young adults [2] and has an boilerplate incidence of two females for each male [one]. The prevalence of MS varies considerably within regions. San Marino and Germany have the highest prevalence in the world (337 and 303 per 100,000, respectively), followed past the United states (288 per 100,000). Reported MS prevalence rates are considerably lower in low- and middle‐income countries (LMICs) than in high-income countries (HICs), but these numbers remain uncertain because of the lack of data [1]. For example, the deficient outdated information indicated an estimation of 1.39 per 100,000 in Shanghai in 2004 and of 54.v per 100,000 in Islamic republic of iran in 2013 [three].
MS is characterized by the loss of motor and sensory functions because of the degeneration of myelin and subsequent loss of the nerves' ability to bear electrical impulses to and from the brain [four, 5]. Consequently, MS can cause an array of symptoms, including upper and lower extremity disabilities, visual disturbances, residue and coordination problems, spasticity, altered awareness, aberrant speech, swallowing disorders, fatigue, bladder and bowel problems, sexual dysfunction, and cerebral and emotional disturbances [iv, six, 7]. These symptoms introduce significant disruptions that negatively affect patients' quality of life, interfere with their productivity [viii], and place societal costs on healthcare systems, caregivers, patients, and their families [9].
Although the clinical course of the affliction is highly variable, MS can exist categorized into two types based on phenotype: relapsing-remitting MS (RRMS) and progressive MS. RRMS, which accounts for 80–85% of initial diagnoses of MS, is characterized past new or recurrent neurologic symptoms (relapses) and stable periods without disease progression (remissions). Relapses are followed by periods of partial or complete recovery. Progressive MS includes secondary progressive MS, with or without relapses, and primary progressive MS [x]. MS progression varies from person to person, and the Expanded Disability Status Scale (EDSS) is used to measure the degree of impairment in neurologic functions [xi]. Available data indicate that health resources consumption and quality of life differ across EDSS levels [12, thirteen].
Price-of-illness (COI) studies are descriptive analyses assessing the economic brunt of a particular health trouble over a divers period of fourth dimension [14]. COI studies inform planning of healthcare services, evaluation of policy options, and prioritization of enquiry [15]; they also provide useful information to foster policy contend [xvi]. COI estimates for MS from numerous countries have been published in recent years, reporting substantial costs per patient [17,18,nineteen,20]. In line with the increasing number of COI studies and their importance, several literature reviews on the topic highlighted the high economical brunt of MS. However, these reviews were published earlier 2010 [sixteen, 21,22,23,24], focused on specific geographical areas [25, 26], were restricted to specific treatments or drugs [27, 28], or were limited to one category of costs, such as intangible costs [29] or informal care [30]. Systematic reviews published after 2010 included studies from HICs [31,32,33,34,35]. Merely one systematic review of MS costs in Latin America, published in 2013 [26], included studies from LMICs, such equally Brazil and Colombia. Although the burden of MS in HICs has been extensively assessed, information on the epidemiology and economic brunt in LMICs remains scarce [36, 37]. Specifically, exploring the COI of MS in LMICs is urgent, as the Atlas of MS, third edition [i], showed that MS registries are increasing in these economies, reflecting a high prevalence of MS. Despite this, no previous systematic review has compiled bear witness on the COI of MS in LMICs. Therefore, this study aims to systematically review the evidence on the COI of MS in LMICs to critically assess the methodologies used, compare toll estimates between countries and by level of disease severity, and examine relevant toll drivers.
Methods
A systematic review was conducted following the standard methods for conducting and reporting systematic reviews (Preferred Reporting Items for Systematic Reviews and Meta-Analyses [PRISMA] statement) [38]. The protocol of the review was registered a priori with the International Prospective Annals of Systematic Reviews (PROSPERO; CRD42019130059).
Data Search
We conducted a systematic search of MEDLINE (Ovid), PubMed, Embase (Ovid), Cochrane Library, National Wellness Service Economical Evaluation Database (NHS EED), EconLit, and CINAHL (EBSCO) to retrieve studies on the COI of MS in LMICs. Records published upward to 26 July 2020 were searched without restrictions on publication yr. To augment the sensitivity of our search strategy, both a free-keyword search and controlled vocabulary were used, such as medical field of study headings, for each of the databases searched. Iii fundamental concepts were considered: multiple sclerosis, cost of illness, AND low- and eye-income countries. For the latter concept, we used the Cochrane filter 2012 (https://epoc.cochrane.org/lmic-filters) and adjusted it to the 2019–2020 Globe Bank nomenclature, which categorizes LMICs every bit low-income, middle-income, and upper middle-income economies. The search strategy was validated by a medical information specialist. An instance of the MEDLINE (Ovid) search strategy is available in the electronic supplementary material.
Searching Other Sources
The search was complemented with astern and forward reference searching. For forrad reference searching, we searched the Web of Scientific discipline database for records citing articles that were included in our review. For astern reference searching, nosotros checked the reference lists of included studies.
Eligibility Criteria
We included original studies published in English, French, and Dutch in peer-reviewed journals containing information on prevalence- or incidence-based cost data for adult patients with MS, from LMICs co-ordinate to the 2019–2020 Globe Bank classification [39]. We excluded editorials, example reports, case series, reviews, and studies reporting on intangible price information, children and adolescents, or any type of MS interventions or economic evaluations.
Selection of Studies
Two reviewers (JD and RR) selected the studies after conducting a calibration exercise by testing eligibility weather to ensure inter-reviewer screening consistency and quality. Commencement, they looked blindly and in parallel for potentially eligible studies by screening the titles and abstracts of the records retrieved by the search. Then, they independently retrieved and evaluated the full texts of references deemed eligible. A screening tool was adult and used for full-text screening. Disagreements were resolved through discussion with other reviewers (MH, IK, and SE).
Data Extraction
Two pairs of authors (JD/RR and JD/IK) independently and in duplicate extracted relevant data from the included studies using a data extraction canvas developed by the five authors and pretested using a calibration exercise before the actual data extraction. Disagreement between reviewers was solved by discussion among all authors to reach consensus. Nosotros extracted data on the study characteristics, analytical framework (eastward.one thousand., bottom-up [BU] vs. top-down [TD] approach), methodology used, near oftentimes reported cost categories, total almanac price per patient, and annual price per patient by severity level and cost ratios.
Data Analysis
The reviewers performed a qualitative synthesis of the data extracted from the included studies. The nature of the data extracted meant a quantitative synthesis was not possible.
It has been reported that the economic brunt of MS includes three price categories: directly medical, straight non-medical, and indirect. Directly medical costs include inpatient care, outpatient care, drugs, diagnostics, surgical interventions, and physician services. Directly non-medical costs include domicile and automobile modifications, informal care provided past family and friends, costs of patients' travel to access healthcare, and most domicile- and community-based services. Indirect costs are losses of production due to curt- or long-term sickness absenteeism, inability alimony, early on retirement considering of health problems, and premature death [9]. Directly medical costs, direct not-medical costs, and indirect costs were reported every bit included in the studies. The virtually oft reported cost categories in MS COI studies were extracted using a checklist compiled by the authors based on reported MS cost units in previous COI [17,eighteen,19] and systematic reviews [31, 34]. The percent of reported price categories was calculated every bit a ratio of the most frequently reported price categories in COI studies of MS. Cost components across MS severity levels were presented by EDSS categories [11]. EDSS scores range from 0 (= normal neurological functioning) to 10 (= death due to MS). EDSS levels as reported past included studies were classified into three conditions based on EDSS score, with scores of 0–3 indicating balmy MS, iv–six.v indicating moderate, and 7–9 indicating severe. To compare study results and toll components per patient overall and by severity of MS, cost estimations per year were converted to $US using World Banking company purchasing power parity [40] and inflated to yr 2019 values using the consumer price index [41]. For studies presenting costs for less than ane year, transformations were made to estimate ane-year costs, bold no seasonal variations in resource use. Regarding studies presenting costs for more than than 1 year, costs were annualized by assuming that costs and healthcare resource consumption were equal during the years of study. For studies only presenting full costs per patient by EDSS classification, the weighted yearly average costs per patient were calculated. When presenting the results, studies were mapped according to the method of calculation, i.e., BU versus TD approaches, to enhance comparisons betwixt studies using the aforementioned methodological approach.
The TD arroyo relies on population-based data such every bit registries, and the BU approach estimates costs based on data from individuals with the affliction and may include questions on breezy care, transportation, and productivity losses not often found in registries [xvi]. The results of a BU study tin start from a subpopulation and be extrapolated to the total population [42].
Dominant cost drivers were determined by identifying the toll category with the highest reported cost per study in general and by EDSS level.
Results
Our initial search, conducted on 5 Oct 2019, retrieved 1099 records, of which merely xi articles [43,44,45,46,47,48,49,fifty,51,52,53] were accounted eligible. The COI study conducted in Russia was reported in two articles [13, 47], and we excluded the commodity that presented the results of 16 by and large high-income European countries [13]. The search was rerun in July 2020, resulting in three additional eligible articles [54,55,56] for a total of xiv studies. Effigy 1 presents the menstruation chart detailing the literature search. Backward and frontward reference searching found no additional studies. As categorized according to the method for computing costs of MS, eight of the 14 identified studies used a BU approach [43,44,45,46,47,48, 54, 55], and half dozen used a TD arroyo [49,l,51,52,53, 56].
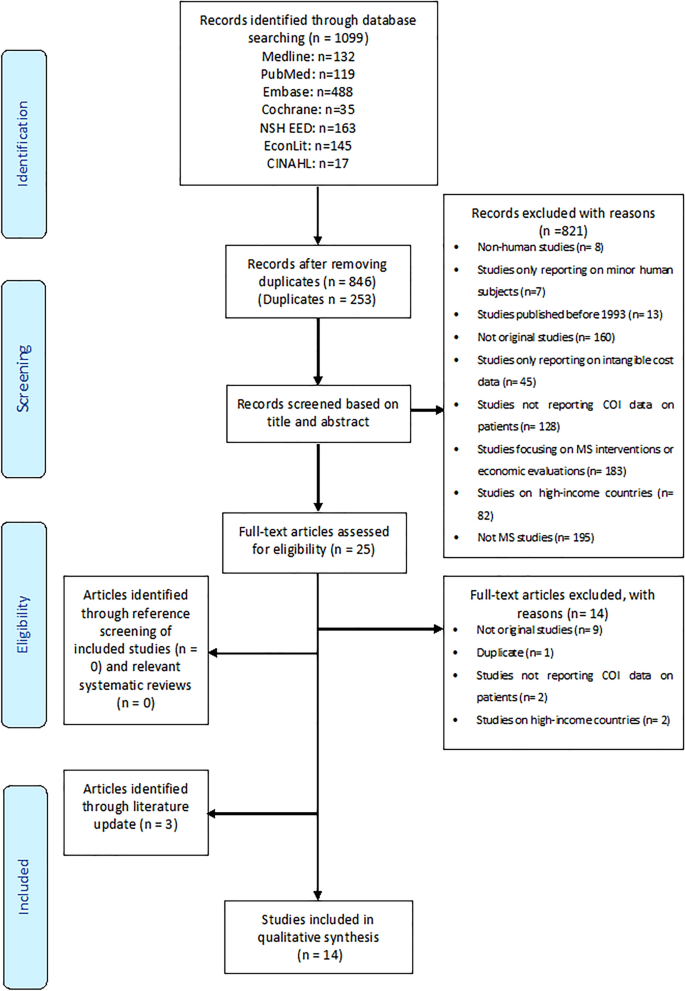
PRISMA flowchart of study selection. COI cost of affliction, MS multiple sclerosis
Characteristics of Included Studies
Table one summarizes the characteristics of included studies. They were conducted in ten countries: 6 in Latin America (Argentina [43], Colombia [50], United mexican states [53], and 3 studies in Brazil [44, 48, 49]), seven in Asia (Turkey [45], Thailand [54], Jordan [51], ii studies in Islamic republic of iran [46, 55], and ii studies in China [52, 56]), and ane in Russian federation [47]. All included studies were published between 2013 and July 2020 and reported on information collected between 2000 and 2018. The number of patients varied from seven in the study by McKenzie et al. [51] to 23,082 in the study by Maia Diniz et al. [49] from Brazil. The mean age of patients ranged between 33.5 [46] and 46.1 years [56]. The percentage of females included varied between 57.0% [51] and 78.7% [44]. The definition of MS was co-ordinate to the RRMS definition [53], a combination of RRMS, secondary progressive MS, chief progressive MS [44,45,46,47,48], or the International Classification of Diseases (ICD) [43, 49,50,51,52, 54, 56]. I study [55] did not written report a definition of MS. All eight BU [43,44,45,46,47,48, 54, 55] studies reported information and costs per patient according to affliction severity using the EDSS nomenclature. Only Chanatittarat et al. [54] used a different EDSS nomenclature (0–two.5, 3–5.v, 6–vii.5, 8–ix.five). Enrollment of patients in all BU studies were up to 1 year, and the timeframe for TD studies varied between ii and 16 years.
Study Methodologies
Study methodologies and costs per patient are presented in Table 2. I BU report [46] did not clearly country whether costs were estimated prospectively or retrospectively; all other BU studies were clearly retrospective and reported prevalence-based COI estimates. 4 BU studies used the societal perspective [43, 44, 47, 54], i [48] used a household and healthcare system perspective, ane [55] used a household perspective, and two [45, 46] did not report the perspective of the analysis. All BU studies measured costs based on a questionnaire. Most BU studies used multiple information sources; two [54, 55] did not study their data sources for costs. Of all BU studies using the human majuscule approach to calculate productivity losses, but da Silva et al. [48] described the impact of productivity losses on patients with MS without converting them into budgetary values. Most of the BU studies used opportunity costs to calculate informal care costs [43,44,45,46,47], whereas the report from Iran [55] did not clearly study the adding method for costing informal intendance.
Five of the TD studies reported prevalence-based COI estimates and were retrospective; Macías-Islas et al. [53] was the exception. Three TD studies [49, 50, 53] used multiple perspectives, ii studies [51, 52] did not report the perspective of the assay, and the perspective used past Du et al. [56] was unclear. TD studies used dissimilar cost measurement tools, i.e., patient records, clinical records, claims, and/or health insurance coverage. Some used a single price listing source, and others used a combination of data sources. The report from China by Du et al. [56] did not study whatever data sources for costs.
Cost Categories
Table 3 presents the detailed cost categories reported in thirteen studies according to the three classifications: direct medical, direct non-medical, and indirect costs. Ane study [51] did non report any cost category so was not included in this table. All but 1 [50] of the 13 studies explicitly reported directly costs for inpatient and outpatient care. All studies reported direct medical costs and, explicitly, the costs of drugs and medical investigations.
All studies reported different costs of healthcare consultation subcategories. Only three studies [43, 47, 48] explicitly reported on all four drug subcategories. In the 13 studies included in Table 3, disease-modifying therapies (DMTs) were the most used drug subcategories, followed by other prescribed medications, and relapse treatments. All except two studies [52, 53] included direct non-medical costs. 5 BU studies [43,44,45, 54, 55] included costs of formal care, informal care, and investments and equipment, whereas TD studies did non include whatsoever costs for formal and informal care. All except 1 [48] BU study reported indirect costs, whereas TD studies did non. Four studies [43,44,45, 55] reported explicitly on productivity losses and absenteeism. Ii studies [44, 47] specifically reported costs of short-term absences, long-term absences, and early retirement. Four BU studies [44, 45, 47, 48] assessed MS illness symptoms and wellness-related quality of life but did non convert them into budgetary values.
The types of price items included varied between the BU studies, whereas TD studies included fewer categories for toll specifications. The largest percent (88%) of included cost categories was reported in the BU report from Russia [47], and the smallest pct (13%) was included in the TD study from Cathay [52]. For example, amid BU studies, the study from Iran [46] had few specified price categories compared with the loftier number of cost categories included in the studies from Brazil [44, 48] and Turkey [45]. Even though the number of cost categories reported past Imani et al. [55] was double that reported in the other report from Islamic republic of iran [46], both studies reported like annual costs per patient.
Multiple Sclerosis (MS) Costs
The total costs per patient with MS ranged between $US463 [52] and 58,616 [43] (twelvemonth 2019 values). Among BU studies, the annual costs per patient were upward to 9 times higher, with an average toll per patient of $US58,616 in Ysrraelit et al. [43] compared with $US6247 in Torabipour et al. [46]. The average pct of direct and indirect costs in BU studies was 89 and 11% of the total costs, respectively. The pct of direct costs varied between 78% [47, 54] and 100% [48] of the total costs, and the highest percentage of indirect costs was 22% for the studies in Russia [47] and Thailand [54].
Comparing of the studies using the societal perspective showed costs per patient were up to three times higher: $US58,616 for Ysrraelit et al. [43] compared with $US15,540 for Kobelt et al. [44].
The two studies from Brazil [44, 48] presented different average costs per patient: the study using a societal perspective and including indirect costs presented more than 40% lower average costs.
The almanac costs per patient in TD studies ranged from $US463 for Min et al. [52] to $US41,514 for Macías-Islas et al. [53].
Price per Patient by Expanded Disability Condition Scale Classification Group
Effigy ii presents the annual cost per patient by EDSS nomenclature group, adjusted to year 2019 $US, and cost ratios past disease severity for BU studies [43,44,45,46,47,48, 55]; one study [54] that did non present costs per EDSS level was excluded. Results of six studies showed that costs per patient increased with disease severity. The highest price ratio was reported in the study from Turkey [45]: i.69 for moderate versus mild disease and 2.87 for astringent versus mild disease. The smallest variation betwixt disease classed equally moderate versus balmy and severe versus mild was reported in the study from Brazil [48], with ratios of one.05 and 1.06, respectively. The calculated hateful cost ratios in BU studies for disease classed as severe versus mild (ane.82) was 26.5% higher than the mean ratio for moderate versus mild illness (1.44). All cost ratios for severe versus mild affliction were college than for moderate versus mild, except for the report from Iran by Imani et al. [55], in which costs for moderate illness were higher than for severe affliction. The range of the price ratios for severe versus mild disease (one.81) was higher than that for moderate versus mild disease (0.64). Costs per patient by EDSS classification varied widely betwixt BU studies, with the widest variation among cost per patient by severe EDSS group, where the highest price was $US77,383 [43] compared with $US9197 [55], the everyman cost for the same nomenclature. Nonetheless, the cost ratios for severe compared with moderate disease for the same studies were almost the same at 1.41 [43] and one.43 [55].
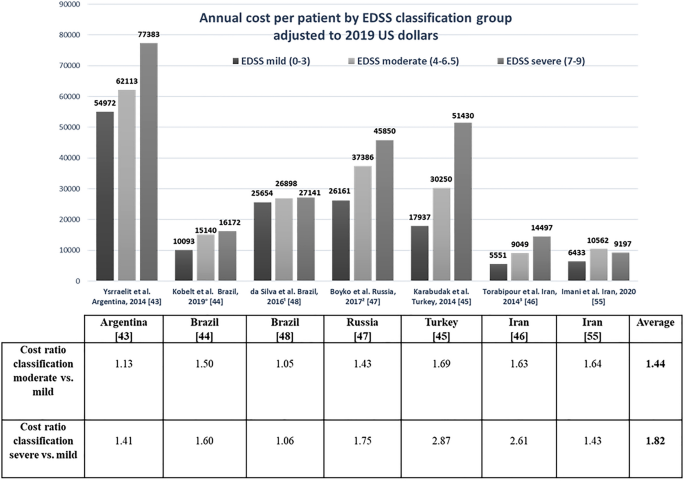
Annual cost per patient past Expanded Disability Status Scale (EDSS) classification group adjusted to $US, year 2019 values, and toll ratios for bottom-up studies. Note that Chanatittarat et al. [54] did non report whatsoever toll past EDSS nomenclature. °Information courtesy of Prof. Gisela Kobelt [44] via personal communication. 1Information about EDSS level was unavailable for two patients in da Silva et al. [48]. twoEDSS information was missing for 20 patients in Boyko et al. [47]. 3To obtain the cost per patient per yr for the study past Torabipour et al. [46] from Iran, nosotros annualized resources used by assuming that nerveless data on resources were representative of patient apply over the whole year
Cost Drivers
Cost drivers differed amidst included studies [43,44,45,46,47,48,49,50,51,52,53,54,55,56], based on the dissimilar levels of cost data specification. Amidst BU studies, DMTs and relapse treatments were the main toll drivers among studies in the mild EDSS group. Although the cost drivers varied more between studies in the moderate EDSS group, relapse treatments and DMTs remained the most dominant cost driver [43, 45, 47, 48, 54, 55], followed past out-of-pocket expenses [44] and domicile care costs [46]. However, the cost drivers varied widely between studies in the severe EDSS grouping, where the drivers beyond studies included relapse treatments and DMTs [43, 48, 55], breezy and formal care [45], rehabilitation [46], and indirect costs [44, 47]. The economic burden increased with greater concrete inability, equally the toll drivers for astringent patients shifted from direct costs to indirect costs. In the TD studies [l,51,52,53, 56], direct medical costs were the dominant toll drivers; these studies did not include indirect costs.
Discussion
This systematic review identified 14 studies investigating the cost of MS in LMICs. All included studies were conducted in upper-middle-income economies, highlighting the absence of COI studies of MS in low-income and depression-middle-income economies. Furthermore, no studies were conducted in Africa. All studies were published between 2013 and 2020 and reported on data collected between 2000 and 2018, suggesting that COI studies of MS are a topic of contempo and increasing involvement in LMICs. The annual costs of patients with MS differed greatly amongst COI studies in LMICs, ranging between $US463 [52] and 58,616 [43] (year 2019 purchasing ability parity values). This could be explained by large methodological variations between the identified studies, and both costs and cost drivers appeared to exist influenced by methodological choices. This MS cost variation could too be attributed to the significant heterogeneity across LMICs, which creates differences in resource use. Furthermore, our study suggested that the full costs increased with disease severity. DMTs and relapse treatments were the master cost drivers for MS in general across studies, but price drivers varied widely across severity levels. Costs of MS drugs were the major cost commuter in lower severity levels, whereas the proportion of direct non-medical costs and indirect costs increased with illness severity.
Methodological and Contextual Differences for Comparability Between Studies
Overall, college costs per patient were reported in Latin American countries [43, 44, 48,49,50, 53], Turkey [45], Russian federation [47], and Thailand [54], whereas lower costs were institute in Islamic republic of iran [46, 55], China [52, 56], and Jordan [51]. Specifically, for the first set of countries, the annual costs per patient ranged from $US15,540 (average cost) in Kobelt et al. [44] to $US58,616 in Ysrraelit et al. [43], and the inclusion of DMTs accounted for twoscore–99% of the average total cost per patient, except for the study in Thailand [54], which did non specify the types and percentage of DMTs included. The studies that did not explicitly include DMTs [46, 51, 52, 56] reported lower annual costs per patient, ranging from $US463 (average cost) in Prc [52] to $US9523 in Hashemite kingdom of jordan [46]. Although the study in Iran by Imani et al. [55] included costs of DMTs, the depression cost per patient ($US7476) could be attributed to the utilize of the household perspective. Amongst the three studies [43, 44, 47] that used a societal perspective, a BU approach, and a relatively mutual methodology to estimate the COI, the absolute costs per patient varied according to the proportion of those costs that were estimated to exist DMT costs. For instance, DMTs accounted for 87.ix% of the total costs per patient ($US58,616) in Argentina [43], 57.ane% in Russia ($US30,358) [47], and twoscore.three% in Brazil ($US15,556) [44]. Although the 3 studies in Brazil [44, 48, 49] used different methodologies, the total costs per patient increased as the percentage of total costs attributable to DMTs increased. These findings propose a positive association between the inclusion of DMTs and the full costs per patient. Directly medical costs, inclusive of DMTs, corresponded to the greatest proportion of the full costs across the 14 included studies. Price drivers were mainly DMTs and relapse treatments and were stable across studies. Notwithstanding, the distribution of cost components varied with severity level. MS drug costs dominated in the mild and moderate EDSS groups, whereas relapse treatments, rehabilitation, indirect costs, and informal care were the cost drivers across studies in the severe EDSS group.
Although absolute costs differed between studies, it appears that the toll ratios between different severity levels across included studies were relatively stable at approximately 1–ane.5 between EDSS balmy and moderate classifications, and 2 between EDSS mild and severe.
Similar to the results of previous systematic reviews of the COI of MS in HICs [23, 24, 31, 34, 35], our findings in LMICs confirm that costs increment with level of disability, as the proportion of straight non-medical costs and indirect costs increased with disease severity. However, in LMICs, indirect costs representing productivity losses announced low and less dominant in the well-nigh severe grouping compared with studies from HICs, where indirect costs represented the majority of the costs. This is primarily considering of the distribution of the sample beyond severity levels. The BU studies included a larger percentage with early disease, representing a larger proportion remaining in the work forcefulness [43,44,45,46,47,48, 55] (the mild EDSS grouping accounted for 40–85% of the samples in included studies). This is in comparison with the findings of Ernstsson et al. [31] in HICs, where the mild EDSS group accounted for 21.3–47.7% of the samples in included studies. Moreover, the proportion of breezy piece of work and shadow economies in developing countries [57, 58], as well as the method used to appraise productivity losses, might have a considerable effect on the costs.
Several important methodological aspects of COI studies are essential to consider in systematic reviews. These include the perspective of the analysis, the scope of costs measured, the analytical framework used to gauge costs (BU vs. TD approach), and the approach used i.e., prevalence- or incidence-based approach [59]. Recent systematic reviews of COI studies of MS in HICs [31, 35] included comparable study characteristics and used methodologies with simply small differences. The majority of COI studies in HICs adopted a societal perspective, primarily a BU approach, and a cross-sectional retrospective assay and included unlike levels of direct and indirect cost data specifications. This enabled systematic reviews [31, 35] to conduct a descriptive assay for studies that reported costs past illness severity (mild, moderate, and severe). The majority of COI studies in HICs are in alignment with local and international health economic guidelines [17, 59,60,61] for conducting and reporting COI studies. However, in our systematic review, the characteristics of, and the methodologies used in, the included studies were highly heterogeneous, especially regarding the perspective adopted, toll data specification, and reporting of costs per severity levels. For example, only 7 [43,44,45,46,47,48, 55] of the 14 studies presented indirect costs per patient likewise as costs per severity level. Thus, detailed and unambiguous reporting of cost units is important equally it enables comparison of methodologies and outcomes of COI studies.
The country-related contexts vary widely in the 3 different economical groups (low, middle, and upper-centre income) in LMICs. The loftier heterogeneity across these countries likely affects the costs of MS because of several land-related factors, including healthcare context-specific issues [62], assessment of healthcare resource consumption, informal care and productivity losses [xxx, 60, 63], reimbursement policies [64], and other cultural and socioeconomic aspects [65]. For instance, transportation costs were higher in the studies from Iran [46, 55] because they were conducted in provinces far outside the capital letter where MS centers are located. Furthermore, informal intendance costs and productivity losses were less dominant in studies from Islamic republic of iran [46, 55] than in those from Argentina [43], Brazil [44], and Russia [47], where formal labor strength participation is more prevalent. Cultural aspects may atomic number 82 to underestimations of informal care; this could be the case in countries such equally Iran where women do not play a significant role in the formal labor marketplace. Furthermore, the definition of breezy care could be perceived differently betwixt countries, which will influence the comparability of these studies [xxx, threescore, 63]. Luz et al. [62] found that the lack of quality local clinical data is an of import technical and context-specific issue when conducting health economic evaluations in LMICs. Thus, this heterogeneity necessitates that methodologies adopted to estimate healthcare resource consumption, informal care, and productivity losses should be well-defined and in alignment with the country's own healthcare system and specifications every bit a marking of the reliability of the COI estimate.
Contextual differences amid countries may pb to large differences in costs per patient [23] and complicate the transferability of economic data across jurisdictions [66,67,68]. Brodszky et al. [69] showed that COI studies in European HICs and upper-center-income economies provided country-specific results, thus limiting the transferability of results. The findings of studies included in this systematic review derived only from upper-middle-income countries, potentially rendering data non-transferable to low-income and low-eye-income economies, where significant variations exist among these groups.
Despite the heterogeneity of the studies included in this systematic review, we used several methodologies to present our findings. Mapping studies according to their method of calculation (BU vs. TD) and using purchasing power parity to convert cost estimates of different currencies to year 2019 $US enhanced the comparability of these studies.
Strengths and Limitations
The strengths and limitations of this review should be considered. We used a highly sensitive search strategy that probable discovered all relevant literature, followed the PRISMA guidelines [38], and registered the written report protocol with the International Prospective Register of Systematic Reviews. Although the burden of MS in HICs has been extensively assessed, to our noesis, this study represents the first systematic review compiling evidence about MS in LMICs. Moreover, we strived to enhance the comparability of the results of the included studies despite their heterogeneity by using recommended and validated methods such as adjusting costs to $U.s., year 2019 values, using Earth Bank purchasing power parity [40] and inflating using the consumer price index [41]; mapping studies co-ordinate to the method of calculation (BU vs. TD); and calculating yearly costs per patient for some studies.
Withal, at that place are also some limitations to this study. First, performing a quality cess of included COI studies was impossible in the absence of a quality assessment checklist. Larg and Moss [15] published a guide to critical evaluation for COI studies simply did not provide a value judgment for each criterion. Therefore, no formal quality assessment of COI studies was conducted using a formal checklist; rather, guidance almost the main elements of methodologies that should be considered in COI studies of MS in LMICs was provided in the give-and-take of this newspaper. Second, this review was restricted to original studies published in English, French, and Dutch. Consequently, one study [37] in Spanish was excluded, and it is possible that other COI studies of MS in different languages could have been missed. Finally, the literature search did not cover governmental reports.
Time to come Directions
Variations between countries precluded extrapolation of data on the COI of MS, and comparisons of costs in absolute terms were unfeasible. Thus, establishing a guideline for conducting and reporting COI studies of MS in LMICs to improve their consistency, reliability, and transferability is needed. Future COI studies of MS in LMICs should include all MS-related toll categories, calculate cost per severity level as MS costs are highly significantly dependent on EDSS categories, and conspicuously define the information source and methodology adopted in alignment with the land's ain healthcare system and specifications. Time to come MS COI studies and systematic reviews should besides pay more attention to low-income, and low-middle-income countries. In addition, there is a general need to develop a consensus quality cess for COI studies with guideline-based interpretations to brand the scoring feasible.
Conclusion
Despite the heterogeneity of studies identified, this systematic review provided a general characterization of the huge economic burden and main cost drivers of MS in LMICs. Cost drivers were mainly DMTs and relapse treatments and were broadly stable across studies. Still, our findings back up that the distribution of cost components varied with the level of disease severity. MS drug costs dominated in lower severity levels, whereas the proportion of directly non-medical costs and indirect costs increased with illness severity. As expected, total costs increased with greater disease severity. Our findings too provide strong support for the concern that at that place are methodological differences and great variations in term of absolute costs per patient across studies and countries, making comparing challenging. However, the price ratios across dissimilar levels of MS severity were similar, making comparisons betwixt studies feasible. This study provided bones and contextual recommendations for futurity researchers on methodological considerations for studies of the COI of MS in LMICs.
References
-
The Multiple Sclerosis International Federation, Atlas of MS, 3rd Edition (September 2020).
-
Dua T, Rompani P, Earth Health Organization, Multiple Sclerosis International Federation, editors. Atlas: multiple sclerosis resources in the world, 2008. Geneva, Switzerland: World Health Organization; 2008.
-
Eskandarieh S, Heydarpour P, Minagar A, Pourmand Due south, Sahraian MA. Multiple sclerosis epidemiology in Eastern asia, South Eastward Asia and South Asia: a systematic review. Neuroepidemiology. 2016;46:209–21.
-
Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:16.
-
Karussis D. The diagnosis of multiple sclerosis and the diverse related demyelinating syndromes: a critical review. J Autoimmun. 2014;48–49:134–42.
-
Noseworthy JH, Rodriguez Grand, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;fifteen:938–52.
-
Zindler East, Zipp F. Neuronal injury in chronic CNS inflammation. Best Pract Res Clin Anaesthesiol. 2010;24:551–62.
-
Judicibus MAD, McCabe MP. The impact of the financial costs of multiple sclerosis on quality of life. Int J Behav Med. 2007;14:3–eleven.
-
Trisolini G, Honeycutt A, Wiener J, Lesesne Due south. RTI International 3040 Cornwallis Road Inquiry Triangle Park, NC 27709 USA: 104.
-
Vollmer T. The natural history of relapses in multiple sclerosis. J Neurol Sci. 2007;256:S5-13.
-
Kurtzke JF. Rating neurologic damage in multiple sclerosis: an expanded inability status scale (EDSS). Neurology. 1983;33:1444–1444.
-
Kobelt One thousand. Costs and quality of life of patients with multiple sclerosis in Europe. J Neurol Neurosurg Psychiatry. 2006;77:918–26.
-
Kobelt G, et al. New insights into the burden and costs of multiple sclerosis in Europe. Mult Scler J. 2017;23:1123–36.
-
Rice DP. Estimating the cost of disease. Am J Public Wellness Nations Wellness. 1967;57:424–forty.
-
Larg A, Moss JR. Cost-of-affliction studies: a guide to critical evaluation. Pharmacoeconomics. 2011;29:653–71.
-
Tarricone R. Cost-of-illness analysis. Wellness Policy. 2006;77:51–63.
-
Svendsen B, Myhr K-Thousand, Nyland H, Aarseth JH. The cost of multiple sclerosis in Norway. Eur J Health Econ. 2012;13:81–91.
-
Karampampa Grand, Gustavsson A, Miltenburger C, Eckert B. Treatment experience, burden and unmet needs (TRIBUNE) in MS study: results from five European countries. Mult Scler. 2012;xviii:seven–15.
-
Palmer AJ, Colman S, O'Leary B, Taylor BV, Simmons RD. The economic impact of multiple sclerosis in Australia in 2010. Mult Scler. 2013;19:1640–half dozen.
-
Reese JP, John A, Wienemann K, Wellek A, Sommer North, Tackenberg B, et al. Economic burden in a German language cohort of patients with multiple sclerosis. Eur Neurol. 2011;66:311–21.
-
Grudzinski AN, Hakim Z, Cox ER, Bootman JL. The economics of multiple sclerosis: distribution of costs and relationship to disease severity. Pharmacoeconomics. 1999;15:229–40.
-
Kobelt G. Economic bear witness in multiple sclerosis: a review. Eur J Health Econ. 2004;5:s54-62.
-
Patwardhan MB, Matchar DB, Samsa GP, McCrory DC, Williams RG, Li TT. Cost of multiple sclerosis by level of disability: a review of literature. Mult Scler. 2005;11:232–9.
-
Orlewska E. Economic brunt of multiple sclerosis: what tin can we learn from price-of-illness studies? Expert Rev Pharmacoecon Outcomes Res. 2006;half dozen:145–54.
-
Adelman Thousand, Rane SG, Villa KF. The cost burden of multiple sclerosis in the United states: a systematic review of the literature. J Med Econ. 2013;16:639–47.
-
Romano M, Machnicki G, Rojas JI, Frider Northward, Correale J. There is much to be learnt nigh the costs of multiple sclerosis in Latin America. Arq Neuro-Psiquiatr. 2013;71:549–55.
-
Sharac J, McCrone P, Sabes-Figuera R. Pharmacoeconomic considerations in the handling of multiple sclerosis. Drugs. 2010;seventy:1677–91.
-
Phillips CJ. The cost of multiple sclerosis and the toll effectiveness of disease-modifying agents in its handling. CNS Drugs. 2004;eighteen:561–74.
-
Wundes A, Brownish T, Bienen EJ, Coleman CI. Contribution of intangible costs to the economical burden of multiple sclerosis. J Med Econ. 2010;thirteen:626–32.
-
Oliva-Moreno J, Trapero-Bertran Chiliad, Peña-Longobardo LM, del Pozo-Rubio R. The valuation of informal care in cost-of-disease studies: a systematic review. Pharmacoeconomics. 2017;35:331–45.
-
Ernstsson O, Gyllensten H, Alexanderson K, Tinghög P, Friberg E, Norlund A. Toll of illness of multiple sclerosis—a systematic review. PLoS Ane. 2016;xi:e0159129.
-
Karampampa K, Gustavsson A, van Munster EThL, Hupperts RMM, Sanders EACM, Mostert J, et al. Treatment feel, burden, and unmet needs (TRIBUNE) in multiple sclerosis study: the costs and utilities of MS patients in The netherlands. J Med Econ. 2013;xvi:939–50.
-
Kolasa K. How much is the cost of multiple sclerosis–systematic literature review. Przegl Epidemiol. 2013;67(i):75.
-
Naci H, Fleurence R, Birt J, Duhig A. Economic Burden of multiple sclerosis: a systematic review of the literature. Pharmacoeconomics. 2010;28:363–79.
-
Paz-Zulueta G, Parás-Bravo P, Cantarero-Prieto D, Blázquez-Fernández C, Oterino-Durán A. A literature review of cost-of-affliction studies on the economic burden of multiple sclerosis. Mult Scler Relat Disord. 2020;43:102162.
-
Risco J, Maldonado H, Luna 50, Osada J, Ruiz P, Juarez A, et al. Latitudinal prevalence slope of multiple sclerosis in Latin America. Mult Scler. 2011;17:1055–9.
-
Romero M, Arango C, Alvis N, Suarez JC, Duque A. Costos de la Esclerosis Múltiple en Republic of colombia. Value in Health. 2011;xiv:S48-50.
-
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA argument. PLoS Med. 2009;6(7):e1000097. https://doi.org/ten.1371/periodical.pmed.1000097.
-
World Banking company Country and Lending Groups. 2020. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. Accessed April 2020.
-
PPP conversion gene, Gdp (LCU per international $). 2020. https://data.worldbank.org/indicator/PA.NUS.PPP. Accessed Apr 2020.
-
Inflation Calculator. 2020. https://cpiinflationcalculator.com/ Accessed Apr 2020.
-
Henriksson F, Jönsson B. The economic cost of multiple sclerosis in Sweden in 1994. Pharmacoeconomics. 1998;13:597–606.
-
Ysrraelit C, Caceres F, Villa A, Marcilla MP, Blanche J, Burgos Grand, et al. ENCOMS: Argentinian survey in cost of illness and unmet needs in multiple sclerosis. Arq Neuro Psiquiatr. 2014;72:337–43.
-
Kobelt G, Teich Five, Cavalcanti M, Canzonieri AM. Brunt and cost of multiple sclerosis in Brazil. PLoS 1. 2019;fourteen:e0208837.
-
Karabudak R, Karampampa K, Çalışkan Z, on behalf of the TRIBUNE Study Group. Treatment experience, brunt and unmet needs (TRIBUNE) in MS study: results from Turkey. J Med Econ. 2015;18:69–75.
-
Torabipour A, Asl ZA, Majdinasab N, Ghasemzadeh R, Tabesh H, Arab K. A study on the straight and indirect costs of multiple sclerosis based on expanded disability status scale score in Khuzestan, Islamic republic of iran. Int J Prev Med. 2014;five:viii.
-
Boyko A, Kobelt Thou, Berg J, Boyko O, Popova Due east, Capsa D, et al. New insights into the burden and costs of multiple sclerosis in Europe: Results for Russian federation. Mult Scler. 2017;23:155–65.
-
da Silva NL, Takemoto MLS, Damasceno A, Fragoso YD, Finkelsztejn A, Becker J, et al. Cost analysis of multiple sclerosis in Brazil: a cross-sectional multicenter study. BMC Health Serv Res. 2016;16:102.
-
Maia DI, Guerra AA, de Lemos LLP, Souza KM, Godman B, Bennie M, et al. The long-term costs for treating multiple sclerosis in a 16-year retrospective cohort study in Brazil. PLoS One. 2018;xiii:e0199446.
-
Muñoz-Galindo IM, Moreno Calderón JA, Guarín Téllez NE, Arévalo Roa HO, Díaz Rojas JA. Health care cost for multiple sclerosis: the example of a Health Insurer in Colombia. Value Wellness Reg Issues. 2018;17:fourteen–20.
-
McKenzie ED, Spiegel P, Khalifa A, Mateen FJ. Neuropsychiatric disorders among Syrian and Iraqi refugees in Jordan: a retrospective accomplice study 2012–2013. Confl Health. 2015;9:10.
-
Min R, Zhang X, Fang P, Wang B, Wang H. Health service security of patients with 8 certain rare diseases: testify from China'south national system for wellness service utilization of patients with healthcare insurance. Orphanet J Rare Dis. 2019;14:204.
-
Macías-Islas MA, Soria-Cedillo IF, Velazquez-Quintana Thou, Rivera VM, Baca-Muro Six, Lemus-Carmona EA, et al. Cost of care according to disease-modifying therapy in Mexicans with relapsing-remitting multiple sclerosis. Acta Neurol Belg. 2013;113:415–20.
-
Chanatittarat C, Chaikledkaew U, Prayoonwiwat Due north, Siritho S, Pasogpakdee P, Apiwattanakul M, et al. Economic burden of Thai patients with inflammatory demyelinating key nervous arrangement disorders (IDCD. Pharm Sci Asia. 2019;46:260–nine.
-
Imani A, Gharibi F, Khezri A, Joudyian N, Dalal K. Economical costs incurred past the patients with multiple sclerosis at different levels of the affliction: a cross-sectional study in Northwest Iran. BMC Neurol. 2020;twenty:205.
-
Du Y, Min R, Zhang 10, Fang P. Factors associated with the healthcare expenditures of patients with multiple sclerosis in urban areas of China estimated by a generalized estimating equation. Expert Rev Pharmacoecon Outcomes Res. 2020;21:137–44.
-
Schneider F, Buehn A, Montenegro CE. Shadow economies all over the earth: New estimates for 162 countries from 1999 to 2007. World Bank policy research working paper. 2010 (5356).
-
Schneider F, Klinglmair R. Shadow economies around the world: What do we know? 2004. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=518526. Accessed xv July 2020.
-
Hodgson TA, Meiners MR. Cost-of-Illness methodology: a guide to electric current practices and procedures. Milbank Meml Fund Q Health Soc. 1982;threescore:429.
-
Clabaugh G, Ward MM. Toll-of-illness studies in the U.s.a.: a systematic review of methodologies used for direct cost. Value Wellness. 2008;11:thirteen–21.
-
Akobundu E, Ju J, Blatt Fifty, Mullins CD. Cost-of-illness studies: a review of current methods. Pharmacoeconomics. 2006;24:869–ninety.
-
Luz A, Santatiwongchai B, Pattanaphesaj J, Teerawattananon Y. Identifying priority technical and context-specific bug in improving the comport, reporting and utilise of wellness economical evaluation in low- and middle-income countries. Health Res Policy Syst. 2018;xvi:4.
-
Onukwugha E, McRae J, Kravetz A, Varga S, Khairnar R, Mullins CD. Cost-of-illness studies: an updated review of current methods. Pharmacoeconomics. 2016;34:43–58.
-
Franken M, le Polain M, Cleemput I, Koopmanschap M. Similarities and differences between five European drug reimbursement systems. Int J Technol Assess Health Care. 2012;28:349–57.
-
Welte R, Feenstra T, Jager H, Leidl R. A conclusion chart for assessing and improving the transferability of economic evaluation results between countries. Pharmacoeconomics. 2004;22:857–76.
-
Zhao F-L, Xie F, Hu H, Li South-C. Transferability of indirect cost of chronic illness: a systematic review and meta-analysis. Pharmacoeconomics. 2013;31:501–8.
-
Sullivan SD. The transferability of economical information: a hard attempt. Value Health. 2009;12:408.
-
Knies S, Severens JL, Ament AJHA, Evers SMAA. The transferability of valuing lost productivity across jurisdictions. Differences between National Pharmacoeconomic Guidelines. Value Wellness. 2010;13:519–27.
-
Brodszky V, Beretzky Z, Baji P, Rencz F, Péntek Thou, Rotar A, et al. Cost-of-disease studies in nine Central and Eastern European countries. Eur J Health Econ. 2019;20:155–72.
Acknowledgements
The authors thank Mrs. Aida Farha, medical information specialist at the American University of Beirut, for her help with the database search and retrieving total-text articles.
Author information
Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to comport this study or prepare this manuscript.
Disharmonize of interest
Jalal Dahham, Rana Rizk, Ingrid Kremer, Silvia K.A.A. Evers, and Mickaël Hiligsmann accept no conflicts of interest that are directly relevant to the content of this article.
Ethics blessing
Non applicable.
Consent to participate
Not applicative.
Consent for publication
Not applicable.
Availability of data and material
All the data supporting the findings of this report (i.e., search strategy and the information extracted from the studies included in the review) are bachelor inside the article and the electronic supplementary cloth.
Lawmaking availability
Not applicable.
Author Contributions
All authors were involved in the concept and blueprint. JD performed the searches. JD and RR conducted the title and abstruse screening. JD, RR, and IK conducted the full-text screening, data extraction, and quality assessment. JD drafted the manuscript. All authors reviewed and edited the manuscript and approved the concluding version of the manuscript.
Supplementary Information
Rights and permissions
Open Access This article is licensed nether a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you requite appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this commodity are included in the commodity's Creative Eatables licence, unless indicated otherwise in a credit line to the textile. If cloth is not included in the article'southward Creative Commons licence and your intended utilize is not permitted by statutory regulation or exceeds the permitted use, yous will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/iv.0/.
Reprints and Permissions
Most this article
Cite this article
Dahham, J., Rizk, R., Kremer, I. et al. Economic Brunt of Multiple Sclerosis in Low- and Heart‐Income Countries: A Systematic Review. PharmacoEconomics 39, 789–807 (2021). https://doi.org/10.1007/s40273-021-01032-7
-
Accepted:
-
Published:
-
Upshot Date:
-
DOI : https://doi.org/ten.1007/s40273-021-01032-seven
Source: https://link.springer.com/article/10.1007/s40273-021-01032-7
0 Response to "The Cost Burden of Multiple Sclerosis in the United States a Systematic Review of the Literature"
Postar um comentário